News & Media
aMDI: Crossing the Development Valley of Death

Dr. Brent Nowak, founder and executive director of aMDI, spoke with Medhealth about all things aMDI. From partnerships to the future of aMDI. They also speak on how Medhealth's matchmaking event helped aMDI form partnerships. Read the full artice here.
How a Grand Valley State institute could impact medical device manufacturing

Brent Nowak, founder and executive director of aMDI, said approximately a dozen undergraduate and graduate students are working on the innovative project. He said 3D additive manufacturing, the process of making three dimensional solid objects from a digital file, is an emerging manufacturing process.
State-of-the-art 3D printing technology from Carbon, Inc., was recently installed in aMDI’s incubator space/lab at GVSU’s Cook-DeVos Center for Health Sciences, located at 301 Michigan St. NE.
3D tech helps GVSU create medical devices
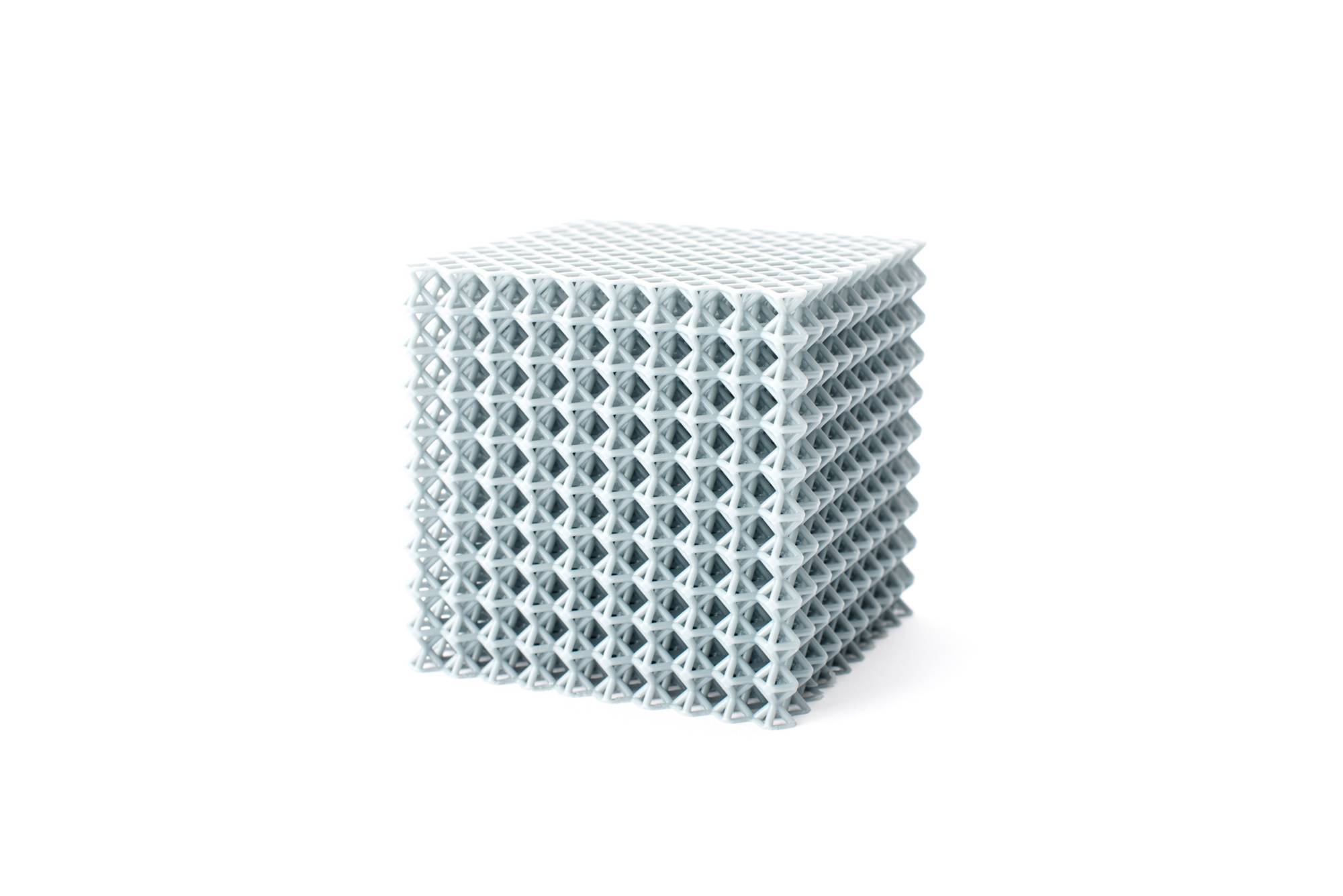
GVSU is one of only six universities in the nation and the only one in the Midwest researching the use of 3D printers to make certain parts. So far, the use of 3D printers has been limited in medical manufacturing.
"What we're doing here is we're studying the scalability of 3D-added manufacturing of medical devices," said Dr. Brent Nowak, the executive director of GVSU's Applied Medical Device Institute.
Applied Medical Device Institute at GVSU awarded $500k grant for medical device manufacturing study

Grand Valley's applied Medical Device Institute (aMDI) has been awarded $500,000 by the Grand Rapids SmartZone to complete an investigation into the viability of using specialized 3D printing technology for medical device production.
The grant will allow aMDI, in collaboration with MediSurge and Carbon, Inc., to create production-grade medical devices that can be safely implanted into and under the skin, which isn't currently possible due to existing materials used for prototyping.
University and Community First to Partner in Midwest to Utilize Innovative 3D Additive Manufacturing Technology to Explore Applications in Medical Device Manufacturing

Grand Valley State University (GVSU) and the Applied Medical Device Institute (aMDI) in Michigan have been awarded half a million dollars for a two-and-a-half year medical 3D printing study. Awarded by the Grand Rapids SmartZone, the fund will be used to determine whether Carbon DLS technology can reduce the cost and time it takes for medical devices to enter the market.
Up-and-coming med device firms exemplify industry’s rise in West Michigan
The design changes, made at Grand Valley State University’s applied Medical Device Institute in Grand Rapids, improved camera images for the surgical trainer, which uses high-definition cameras, LEDs, and a software package that creates intricate, X-ray-like images displayed on a tablet or computer screen. Real-time imaging and web connectivity allow users to conduct training sessions through webinars, as well as reduces users’ exposure to X-rays.
Accelerating Development: Medical device firms turn to GVSU institute for R&D help

As Sterilogy now works to tweak the design to make it more “manufacturing-friendly” and prepares for beta-testing the hand-sanitizer system in 2019, Zaima credits the company’s progress to aMDI.
“We couldn’t have gotten to where we are today without aMDI,” said Zaima, Sterilogy’s co-founder and president. “aMDI was able to take our statement of work and make it into a working prototype, make it into reality.”
GVSU Students Develop Hand Sanitizing Product For Health Care Workers

Jonathon Vinsko, a computer engineering graduate assistant, said it was a valuable experience to be able to take Sterilogy’s concept and build it from the ground up.
“All three devices work together to ensure that health care workers are complying with health standards,” Vinsko said. “When a nurse or doctor walks up to a new patient or away from a patient, they are required to sterilize their hands. Sometimes people forget, so this system serves as a quick reminder.”
GVSU group develops system to reduce hospital infections and increase sanitation

Zaima enlisted the help of aMDI in spring 2017 to design, build and test a series of prototypes for the hand hygiene system, which includes three separate devices. The personal sanitizer unit (PSU) is a body-worn device that dispenses the foam sanitizer; the zone alert emitter unit is attached to a patient's bed and communicates with the PSU to remind health care workers to sanitize; and the base station unit is placed at a central location, like a nurse's station, and uploads data from PSUs when they are in close range.
aMDI & Sterilogy develop hand hygiene system

Grand Valley's applied Medical Device Institute (aMDI) worked with Sterilogy to develop the hand hygiene compliance system with a goal to reduce hospital infections. The three-device system was designed for nurses, doctors and other health care workers who come in direct contact with patients.
"Every day, about 250 people across the country die from hospital infections, and over half of infections are caused by direct contact," said Hal Zaima, President of Sterilogy, located in Bloomfield Hills. "It results in more than $30 billion in unnecessary costs to hospitals."
aMDI Organizes Training on SBIR/STTR Grants

applied Medical Device Institute (aMDI) organized a half-day long training on July 12, to introduce a small invited group to the SBIR/STTR program and how to leverage the grant dollars to bring their product to market. The Federal SBIR/STTR programs award over $2.6 billion in high risk R&D funding annually to qualified small businesses. The process for applying can be complex, but with training and assistance, SBIR/STTR can become a catalyst for launching a company.
aMDI Leads Panel at the First Ever MedDevice Forum in Grand Rapids

MedDevice Forum, a brand new medical device conference, debuted on May 22, 2018, in Grand Rapids, Michigan. Hosted by Michigan State University’s College of Human Medicine, the purpose of this event was to discuss the most pressing issues medical device companies are facing and the growing role of academia in medical device research and development. Brent M. Nowak, Ph.D., Executive Director of applied Medical Device Institute (aMDI), moderated a panel named: Pathway to Innovation—Academia’s Role in Device Development. The panel discussed the rising inclination amongst medical device manufacturers to enable universities to serve as their R&D facility. The panelists expressed their views on the benefits of the arrangement and where they expect it to go in the future.
applied Medical Device Institute (aMDI) to Host Additional Lunch and Learn Event

Accelerating the transfer of new technologies from Michigan’s institutions of higher education, hospital systems and nonprofit research centers to the private sector, is at the heart of several early stage programs supported by the Michigan Economic Development Corporation Entrepreneurship & Innovation initiative.
This discussion will review several MEDC programs that support the commercialization of medical device and healthcare innovation from proof of concept, to translational research, to startups and industry collaboration. Information will provide details on matching funding available, support services such as mentors in residence and industry collaborations.
applied Medical Device Institute (aMDI) Awarded Additional Local Funding

The aDMI has been awarded a grant from the Grand Rapids SmartZone Local Development Finance Authority (LDFA) to support student, faculty and staff projects that focus on the development of medical devices. Funds will allow those involved with the aMDI to work alongside two local technology companies in developing these devices, and creating plans for their manufacturing and sale. The aMDI continues to serve as an example of a well-conceived, efficient launching ground for innovation in medical technology.
How Does The GVSU aMDI Help Entrepreneurs Negotiate The FDA Approval Process?

What does it take for a medical device to get approved by the Federal Drug Administration for commercialization? Brent Nowak, PhD, executive director of the Applied Medical Device Institute of Grand Valley State University, explains how the process works.
Nowak is interviewed on this segment of M2 TechCast by Keith Brophy, state director of the Michigan Small Business Development Center, part of a monthly series where Brophy highlights companies and resources available for entrepreneurs through this SBA-backed organization.
applied Medical Device Institute launches female catheter
An external catheter for female hospital patients may be available in the next year, and it will have been created and developed in Grand Rapids.
Brent Nowak said the uCol is one of a handful of medical devices the new applied Medical Device Institute, where he serves as executive director, is working on with its partners.
The aMDI has been operating for just over a year but became an official unit of Grand Valley State University earlier this year. It is housed on the fifth floor of the Cook-DeVos Center for Health Sciences.
First applied Medical Devices Institute comes to GVSU
Grand Rapids is known for many things. From craft beers to diverse museums and a continually growing healthcare industry, businesses related to these industries are moving to West Michigan in search of a new and engaged market.
After being approved a year ago following increased interest from the community, Brent Nowak, director of the first applied Medical Devices Institute (aMDI) in West Michigan has been working to serve the healthcare community of the greater Grand Rapids area.
Read more about the healthcare community in the Greater Grand Rapids area


