GVSU Technologies Available for License
Grand Valley State University’s Technology Commercialization Office (TCO) facilitates the transfer of GVSU innovations to the market. The University has several innovations available for licensing and commercialization that represent multiple industry sectors.
Available technologies are subject to change depending on ongoing licensing or commercialization deals. For additional information about any of the technologies, please email [email protected], or connect with Linda Chamberlain.
A Novel, Energy-Saving System for Demand-Control Ventilation in Applications of Varying Occupancy
Developer of ventilation system designed for indoor air quality monitoring. The company offers centralized sensing technology that delivers accurate data on a scalable platform with an analytics dashboard, enabling schools, healthcare, and commercial industries to manage ventilation requirements.
This technology offers a state-of-the-art method for monitoring indoor air quality. The “centralized” sensing hub delivers accurate and timely data on a scalable platform. When coupled with a demand-control ventilation strategy, provides a state-of-the-art solution to insure an area is neither over nor under ventilated, maintaining a healthy building environment conducive to learning and working. Better control of building ventilation also reduces energy and building maintenance costs.
Product now for sale: www.antrum.com
Sensing Technology
Licensed to Antrum Corporation, July 2020
Cough Assist Device
Patients with neuromuscular diseases require a cough assist device to dislodge and remove mucus from their lungs. Therapy consists of bursts of pressure/vacuum or vibration to clear the airway and simulate a cough. Current solutions are expensive and non-portable. This invention has delivered a low cost, nonelectric cough assist device with equivalent therapy to the current device.
In collaboration with Corewell Health
Available for License

External Urinary Device
The external urinary collection device for females was designed as an alternative to the use of indwelling catheters and briefs. The device helps reduce the occurrence of catheter associated urinary infections.
In collaboration with Spectrum Health Innovations
Licensed to SBE Medical, July 2020
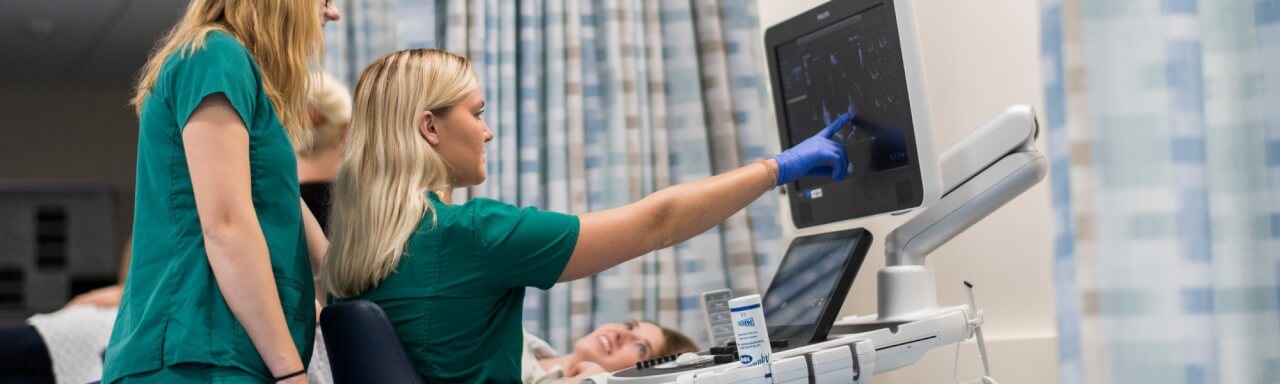
Labor Assist Device
The Labor Assist device is an easily deployable, patient-controlled birthing bed accessory that utilizes a mechanical assist to restore a laboring mother’s independence and lessen the risk of injury for healthcare providers. The device is easily integrated into existing hospital birthing beds.
In collaboration with Corewell Health East (Beaumont)
Check out the Labor Assist Device Prototype Video (2160p)
Available for License
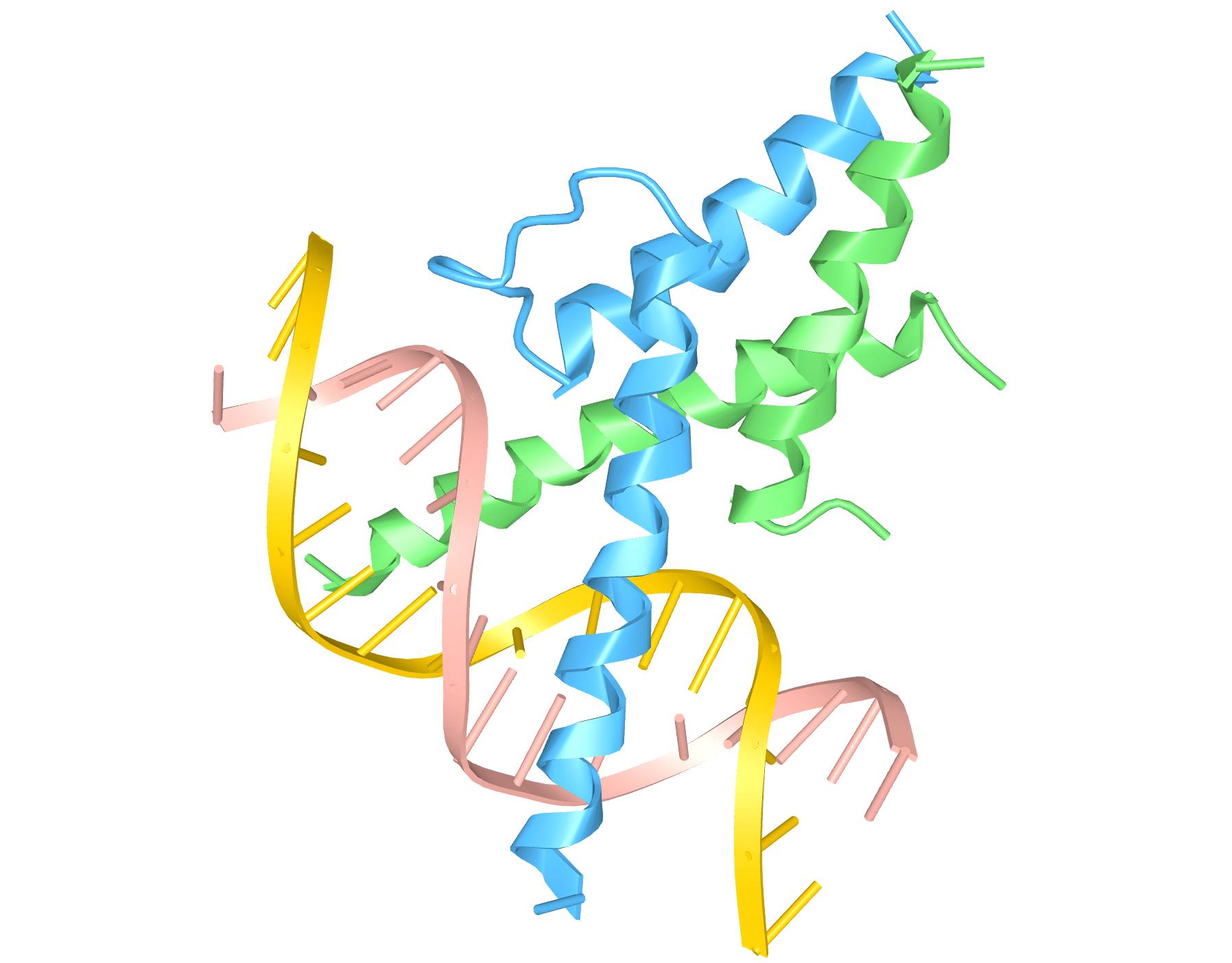
Mutant Nato3 Polypeptides: Manufacture of Dopamine Neurons
PM-Nato3 is a novel polypeptide tool that promises to replace use of costly reagents and simplify the conversion of human stem cells (hSC) into dopamine (DA) neurons. DA neurons are lot in Parkinson's disease (PD) and affected in schizophrenia and other disorders, and therapeutics in the realm represent a growing market of 6.7 billion USD. Products would include cell lines(s) expressing PM-Nato3 polypeptides that have a dopaminergic phenotype to be used for 1) preclinical drug recovery for PD, schizophrenia and other disorders and 2) transplantation into brains of patients with PD. Our technology could be delivered as a lentivirus or cell permeable polypeptide to simplify a time consuming (35 day) and technically difficult process that relies on costly and degradable biological reagents.
Available for License
SimPull Patient Transfer
This invention addresses the challenges in performing lateral patient transfers. The device supports making lateral transfers under the operation of just one clinician, without having to exert any force, or manipulate patients in any way.
In collaboartion with Corewell Health.
Product now for sale: www.thepatientcompany.com
Licensed to the Patient Company, January 2020
[1572016853].jpg)

Snake Pen
Envenomation by snakebite is a relevant public health concern in tropical and subtropical regions of the world, leading the World Health Organization to classify it as a top priority Neglected Tropical Disease in 2017. Globally, three million people are bitten by poisonous snakes, leading to an estimated 400,000 amputations and 125,000 fatalities annually. Current therapy requires the use of intravenous multi-valent antivenin administered in a hospital setting, an approach that too often involves significant delay in treatment leading to increased morbidity and mortality. This invention includes design of a field-based delivery device that auto-injects venom antidotes to reduce negative outcomes immediately after envenomation.
Available for License