Brad Wallar Research Interests
β-lactams, such as the penicillins and cephalosporins, are the most widely prescribed class of antibiotics. While bacterial infections have been effectively treated with β-lactam antibiotics for many decades, resistance to these drugs has been steadily growing. In response to their extensive use and misuse, bacteria acquired the ability to destroy β-lactams, allowing many resistant strains to develop. The most widespread resistance mechanism to β-lactams is through the β-lactamase enzymes. These enzymes are expressed by a growing number of bacteria and render a wide range of antibiotics inactive. As a result, antibiotic resistance has emerged as one of the leading public health crises of the 21st century.
[1535992727].jpg)
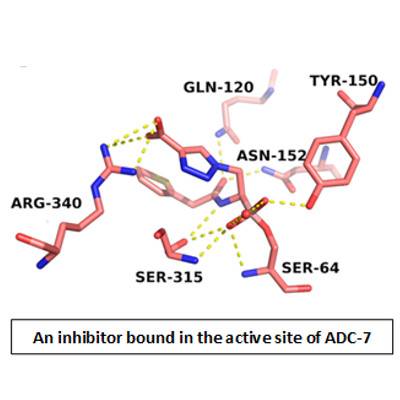
Classified as a “threat level serious” pathogen by the Centers for Disease Control, the multidrug-resistant Acinetobacter baumannii presents one of the greatest challenges to contemporary antimicrobial chemotherapy. Much of the resistance to cephalosporins derives from the expression of the class C β-lactamase enzymes, known as Acinetobacter-derived cephalosporinases (ADCs). While it is possible to inactivate β-lactamase enzymes by inhibitor molecules, current b-lactamase inhibitors are structurally similar to β-lactam antibiotics and are not effective against the class C cephalosporinases.
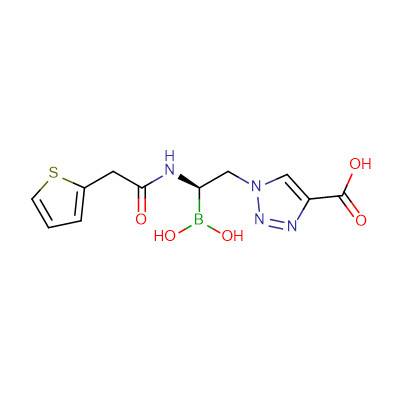
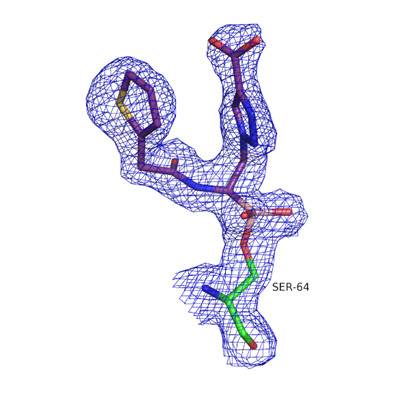
Therefore, in collaboration with researchers from GVSU, Case Western Reserve, and Università di Modena e Reggio Emilia (Italy), a new class of boronic acid transition state inhibitors (BATSIs) have been synthesized and shown to be effective at inhibiting ADC enzymes. Using a combination of enzyme kinetics, site-directed mutagenesis, and X-ray crystallography, the Wallar lab continues to study a group of BATSIs in order to identify the most effective inhibitor of ADC β-lactamase activity.