Individual- and Population-Level Effects of Antibiotics on Rotifers
James N. McNair and Adriana Belém de Araújo, Annis Water Resources Institute, Grand Valley State University
Overview
Antibiotics are an important class of trace environmental contaminants. Many of these compounds are produced by common molds and bacteria and therefore occur naturally in the environment. However, enormous quantities of antibiotics are now produced and used by humans. Applications include drugs in human and veterinary medicine, growth promoters in intensive livestock production, agricultural pesticides, and additives in many consumer and personal care products.
Antibiotics enter the environment from multiple sources, including excretion by humans, pets, and farm animals, spraying of agricultural crops, chemotherapy and antibiotic food additives in net pen aquaculture, and inappropriate use and disposal by humans (Halling-Sørensen et al., 2002; Boxall et al. 2003; Thiele-Bruhn, 2003). Once in the environment, they enter natural water bodies via hydrologic transport in agricultural areas, passage through municipal wastewater treatment plants, and a variety of other pathways. Cursory surveys of streams in the U.S. and U.K. have found human and veterinary antibiotics at individual concentrations of up to roughly 1.0 μg/L (Watts et al., 1982; Lindsey et al., 2001; Kolpin et al., 2002), though higher concentrations probably occur (e.g., in streams draining agricultural fields after spreading of manure).
Quantitative information regarding ecological effects of antibiotics on aquatic organisms is scarce. Among prokaryotes, Backhaus & Grimme (1999) demonstrated inhibition of the marine luminescent bacterium Vibrio fischeri by tetracycline and ofloxacin at concentrations on the order of 1 μg/L, and Holten Lützhøft et al. (1999) and Halling-Sørensen (2000) demonstrated inhibition of the freshwater cyanobacterium Microcystis aeruginosa by amoxicillin, benzylpenicillin, spiramycin, streptomycin, and tiamulin at concentrations on the order of 1 μg/L. Among eukaryotes, the lowest reported inhibitory concentrations are on the order of 1 μg/L for the unicellular green alga Pseudokirchneriella subcapitata (formerly Selenastrum capricornutum) exposed to clarithromycin (Isidori et al., 2005), and 100 μg/L for the freshwater cladoceran Ceriodaphnia dubia exposed to oxytetracycline and the freshwater rotifer Brachionus calyciflorus exposed to ofloxacin (Isidori et al., 2005). Inhibitory concentrations of 1000 μg/L (1 mg/L) or higher have been reported for the freshwater cladoceran Daphnia magna exposed to bacitracin (Dojmi di Delupis et al., 1992), the brine shrimp Artemia salina exposed to sulfadimethoxine (Brambilla et al., 1994), and the guppy Lebistes reticulatus exposed to furazolidone (Canton & Van Esch, 1976). Thus, inhibitory concentrations reported for unicellular organisms (especially prokaryotes) lie within the range of antibiotic concentrations observed in natural water bodies, while inhibitory concentrations reported to date for aquatic invertebrates and vertebrates are much higher.
Several problems limit the usefulness of available ecotoxicity information for assessing ecological risks of antibiotics. Very few species have been assessed for any individual antibiotic, most studies only report concentrations at which extremely large effects occur (usually a 50 % reduction in survival or population growth), most studies of aquatic invertebrates and fish employ exposure durations that are very short compared to the typical lifespan of adequately maintained test organisms (longer test durations typically yield effects at lower concentrations), and most studies of aquatic invertebrates and fish assess only effects on survival rather than including sublethal effects on reproduction, growth, or behavior (which typically occur at lower concentrations than do survival effects). As a result of these problems, the number of species for which data are available is insufficient to permit a credible estimate of the distribution of species sensitivities for any antibiotic, and data for most species and antibiotics that have been assessed do not provide a plausible estimate of the lowest concentration at which ecologically meaningful effects first arise.
Rotifers are excellent test organisms for addressing some of the problems with available antibiotic ecotoxicity data for aquatic invertebrates. These organisms play a central role in the dynamics of freshwater and coastal marine ecosystems, are easy to culture in the laboratory, have much shorter lifespans than more-widely used microcrustacean test species such as Daphnia magna and Ceriodaphnia dubia, are much smaller than these same microcrustacean species and therefore can be cultured in much smaller volumes of medium, and can readily be obtained as resting eggs from commercial suppliers (Preston & Snell, 2001; Preston et al., 2000; Snell & Carmona, 1995; Janssen et al., 1994; Wallace & Snell, 1991). Their short lifespan (roughly 2 weeks at 25°C) makes full-lifespan test durations much more feasible than with cladocerans or larger invertebrates, and the commercial availability of resting eggs and small volume of culture medium required per test subject (100 μL) permit relatively high degrees of replication. These properties are expected to increase the sensitivity of tests.
The objective of the present study was to assess the chronic toxicity of three common antibiotics to two widely occurring freshwater and brackish-water rotifers. Specifically, we conducted full-lifespan survival and reproduction tests to determine the inhibitory effects of streptomycin sulfate (an aminoglycoside), tetracycline hydrochloride (a tetracycline), and tylosin tartrate (a macrolide) on the freshwater rotifer Brachionus calyciflorus and brackish-water rotifer B. plicatilis. Our results provide the first information regarding toxic effects of these antibiotics on rotifers.
Experimental Procedures
Effects of each antibiotic (streptomycin sulfate, tetracycline hydrochloride, and tylosin tartrate) on asexual reproduction, lifespan, and several demographic parameters (Malthusian parameter, net reproduction rate, and three key properties of the net maternity function) were assessed at five nominal concentrations (ranging from 5.6 to 2000 mg/L) and a control (without antibiotics). Test animals were obtained by hatching resting eggs and were individually cultured in wells with 100 μL test medium and food at a fixed concentration ( Chlorella vulgaris at 3×106 cells/mL for B. calyciflorus, and Nannochloropsis oculata at 7×106 cells/mL for B. plicatilis). The number of offspring from each test animal was counted daily, and lifespan was recorded at death. Lowest Observed Effect Concentrations (LOECs) were determined for reproduction and lifespan; 1, 10, 25, and 50 % Inhibitory Concentrations (ICs) and 95 % confidence intervals were estimated for all endpoints.
Statistical Methods
Effects of antibiotics on several individual- and population-level properties were assessed, including lifespan, lifetime reproduction, and Malthusian parameter (dominant eigenvalue of the Bernardelli-Leslie matrix, or the theoretical asymptotic geometric growth rate of an isolated population with time-invariant survival and fecundity schedules). Lowest Observed Effect Concentrations (LOECs) were determined for individual-level endpoints (lifetime reproduction and lifespan); 1, 10, 25, and 50 % Inhibitory Concentrations (IC1, IC10, IC25, and IC50) and 95 % confidence intervals were estimated for all endpoints.
For each rotifer species, the lifespan LOEC for each antibiotic was determined by finding the lowest test concentration that produced a statistically significant ( p d 0.05) reduction in lifespan, and similarly for the lifetime reproduction LOEC. Data properties (variance heterogeneity and non- normality) prevented use of standard parametric statistical methods. All comparisons of treatments and controls were instead conducted using two-sample bootstrapping. In each case, the null hypothesis was that the control and treatment means were the same; the alternative hypothesis was that the treatment mean was less than the control mean. The resulting p values for each antibiotic (one p value for each comparison of treatment and control) were adjusted to control the experiment-wise error rate using Holms method (Holm, 1979), which is more powerful than the Bonferroni method but valid under equally general conditions.
IC x values were estimated by a combination of nonlinear regression and bootstrapping. A suitable concentration-response function (CRF) was chosen for each test endpoint by fitting all regression models from a master list to data consisting of the test concentrations and the corresponding endpoint means, repeating the procedure several times, and then examining the sum of squared errors and the consistency of the parameter estimates across repetitions (see example in Figure 1). IC x ( x = 1, 10, 25, 50) values for each endpoint were determined by case-based bootstrapping stratified by concentration, with the CRF being re- fit to each bootstrap sample and the implied IC x values calculated. The mean, median, and 95 % confidence interval for each ICx were determined from these bootstrap distributions. For additional details, see Araujo & McNair (2007).
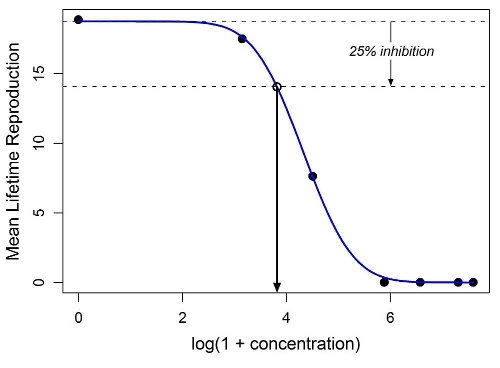
Figure 1. Example of a fitted CRF (in this case, a log Gaussian function fitted to mean lifetime reproduction data for B. calyciflorus exposed to tetracycline). The lower horizontal dashed line represents 25 % inhibition of mean lifetime reproduction. The concentration at which this line intersects the CRF is the corresponding IC25.
Results
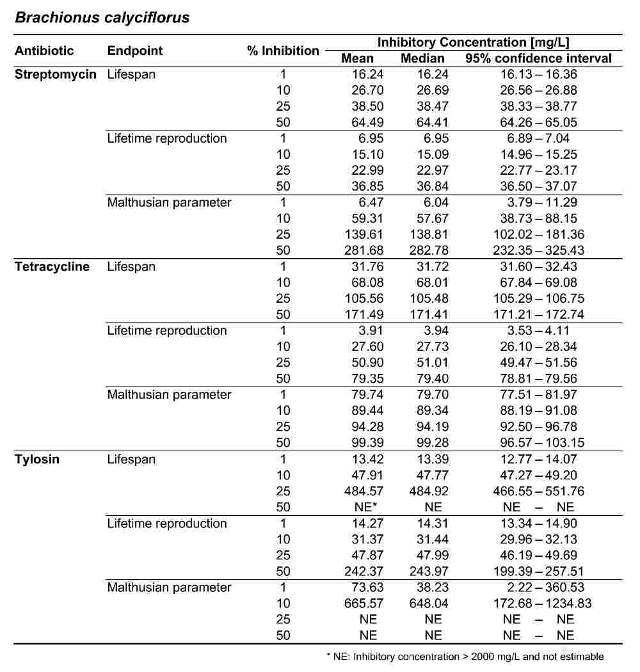
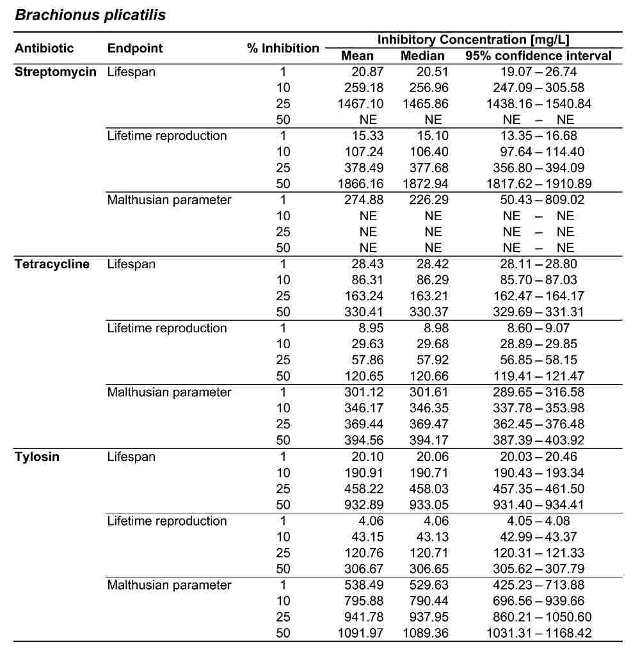
Figure 2 shows the response of B. calyciflorus age-specific survival and fecundity to streptomycin exposure. Broadly similar patterns were observed for B. calyciflorus and the other antibiotics. The main results are summarized in Tables 1 and 2. All B. plicatilis LOECs were 90 mg/L (reproduction and lifespan for all three antibiotics). For B. calyciflorus, tylosin LOECs were 90 mg/L for reproduction and lifespan, tetracycline LOECs were 5.6 mg/L for reproduction and 90 mg/L for lifespan, and streptomycin LOECs were 5.6 mg/L for both reproduction and lifespan. All LOECs and ICs were well above 10 μg/L, which is considered the maximum antibiotic concentration likely to occur in natural water bodies. Additional details and discussion can be found in Araujo and McNair (2007).
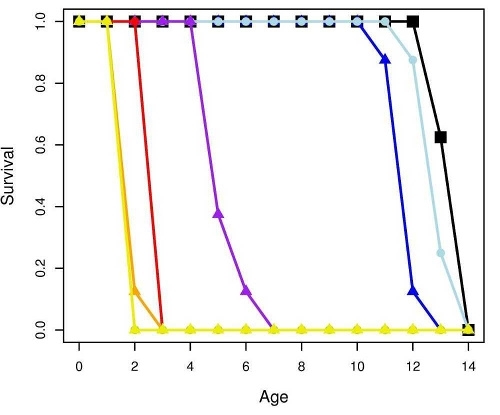
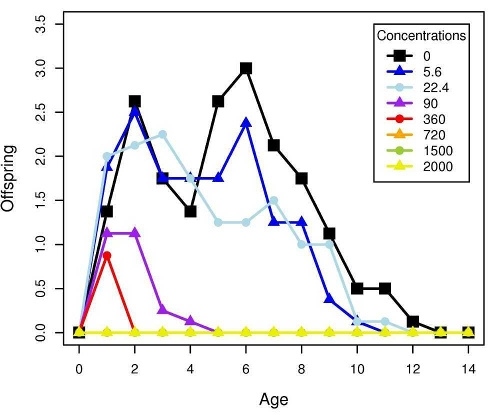
Figure 2. Age-specific survival and fecundity schedules for B. calyciflorus exposed to streptomycin. Fecundity at age x (upper panel) is the average number of live offspring produced by mothers of age x in a given treatment or control. Survival to age x (lower panel) is the proportion of the initial eight test subjects in a given treatment or control that were still alive at age x days. Streptomycin concentrations are in mg/L.
Table 1. Summary of LOECs and NOECs for both rotifer species and all three antibiotics tested.
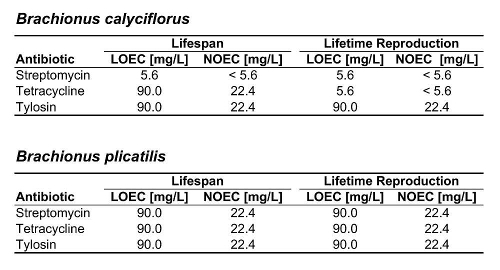
Table 2. Inhibitory concentrations for B. calyciflorus and B. plicatilis, in mg/L. For each antibiotic and response variable, the mean, median, and 95 % confidence interval for the IC1, IC10, IC25, and IC50 are shown. NE indicates that the IC was greater than the maximum test concentration (2000 mg/L) and therefore not estimable.
Conclusions
The main conclusions of the study are as follows:
- The rotifer species tested showed different sensitivities to antibiotic exposure, with B. calyciflorus being the more sensitive of the two species.
- B. calyciflorus was more sensitive to streptomycin than to the other test antibiotics, while B. plicatilis was most sensitive to tetracycline.
- Lifetime reproduction (asexual) and lifespan decreased as antibiotic concentrations increased.
- Overall, the Malthusian parameter was the least sensitive endpoint and lifetime reproduction the most sensitive.
- With B. calyciflorus, streptomycin LOECs for lifespan and lifetime reproduction were lower than the corresponding IC1 values. For most other antibiotics and endpoints, IC1 and IC10 values were lower than LOECs.
- With B. plicatilis, LOECs for lifetime reproduction and lifespan were higher than IC1 values for all antibiotics but were not consistently higher or lower than IC10 values.
- Based on a recent survey of U.S. streams conducted by the U.S. Geological Survey (USGS), it appears that freshwater antibiotic concentrations typically are less than 10 μg/L (though much higher concentrations probably occur; e.g., in streams draining agricultural fields after spreading of manure). In contrast, all LOECs and ICs in the present study were greater than 1 mg/L (1000 μg/L). Taken together, these results suggest that the antibiotics tested are unlikely to have meaningful effects on B. plicatilis or B. calyciflorus in most U.S. streams and associated estuarine water bodies. It remains possible, however, that test protocols which extend over multiple generations (as in bacterial and algal protocols), include density-dependent effects, or include secondary effects on rotifers of primary effects on their bacterial or algal food sources may prove more sensitive and reveal effects at much lower concentrations.
References
Araujo, A.B. & McNair, J.N. 2007. Individual- and population-level effects of antibiotics on the rotifers, Brachionus calyciflorus and B. plicatilis. Hydrobiologia 593: 185–199.
Backhaus T. & Grimme, L.H. 1999. The toxicity of antibiotic agents to the luminescent bacterium Vibrio fischeri. Chemosphere 38: 3291–3301.
Brambilla, G., Civitareale, C. & Migliore, L. 1994. Experimental toxicity and analysis of bacitracin, flumequine, and sulfadimethoxine in terrestrial and aquatic organisms as a predictive model for ecosystem damage. Quimica Analitica 13: 114–118.
Boxall, A.B.A., Kolpin, D.W., Halling-Sørensen, B. & Tolls, J. 2003. Are veterinary medicines causing environmental risks? Environmental Sciences and Technology 37: 286A–294A.
Canton, J.H. & Van Esch, G.J. 1976. The short-term toxicity of some feed additives to different freshwater organisms. Bulletin of Environmental Contamination and Toxicology 15: 720–725.
Dojmi di Delupis, G., Macri, A., Civitareale, C., & Migliori, L. 1992. Antibiotics of zootechnical use: effects of acute high and low dose contamination on Daphnia Magna Straus. Aquatic Toxicology 22: 53–60.
Halling-Sørensen, B. 2000. Algal toxicity of antibacterial agents used in intensive farming. Chemosphere 40: 731–739.
Halling-Sørensen, B., Nielsen, S.N. & Jensen, J. 2002. Environmental Assessment of Veterinary Medicines in Denmark. Copenhagen: Danish Environmental Protection Agency.
Holm, S. 1979. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6: 65–70.
Holten Lützhøft, H.C., Halling-Sørensen, B. & Jorgensen, S.E. 1999. Algal toxicity of antibacterial agents applied in Danish fish farming. Archives of Environmental Contamination and Toxicology 36:1–6.
Isidori M., Lavorgna, M., Nardelli, A., Pascarella, L. & Parrella, A. 2005. Science of the Total and Environment 346: 87–98
Janssen, C. R., Persoone, G. & Snell, T. W. 1994. Cyst-based toxicity test. VIII. Short-chronic toxicity tests with the freshwater rotifer Brachionus calyciflorus. Aquatic Toxicology 28:243–258.
Kolpin, D.W., Furlong, E.T., Meyer, M.T., Thurman, E.M., Zuagg, S.D., Barber, L.B. & Buxton, H.T. 2002. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 19992000: a national reconnaissance. Environmental Science and Technology 36: 1202–1211.
Lindsey, M.E., Meyer, M. & Thurman, E.M. 2001. Analysis of trace levels of sulfonamide and tetracycline antimicrobials in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry. Analytical Chemistry 73: 4640–4646.
Preston B.L. & Snell, T. W. 2001. Full life-cycle toxicity assessment using rotifer resting egg production: implications for ecological risk assessment. Enviromental Pollution 114: 399–406
Preston B.L., Snell, T.W, Robinson, T.L. & Dingmann, B.J. 2000. Use of the freshwater rotifer Brachionus calyciflorus in a screening assay for potential endocrine disruptors. Environmental Toxicology and Chemistry 19: 2923–2928.
Snell T. W. & Carmona, M. J. 1995. Comparative toxicant sensitivity if sexual and asexual reproduction in the rotifer Brachionus calyciflorus. Environmental Toxicology 14: 415–420.
Thiele-Bruhn, S. 2003. Pharmaceutical antibiotic compounds in soils a review. Journal of Plant Nutrition and Soil Science 166: 145–167.
Wallace, R. L. & Snell, T. W. 1991. Rotifera. Pp. 187248 in Thorp, J. H. & A.P. Covich (eds.). Ecology and Classification of North American Freshwater Invertebrates. Academic Press, New York, 950 pp.
Watts, C.D., Crathorne, B., Fielding, M. & Killops, S.D. 1982. Non-volatile organic compounds in treated waters. Environmental Health Perspectives 46: 87–99.
