Dave Leonard - Research
Research Interests
Bacterial Resistance to Lactam Antibiotics
Lactamase Enzyme Structure/Function
We are currently studying the breakdown of lactam antibiotics such as ampicillin and doripenem by the Class D lactamases OXA-1, OXA-23 and OXA-24. Class D lactamases are known to use a serine-based covalent catalysis mechanism. They are also known to require the carboxylation of the epsilon amino group of an active site lysine. This carboxylated lysine plays the role of general base, deprotonating the active site serine and preparing it for attack of the lactam carbonyl.
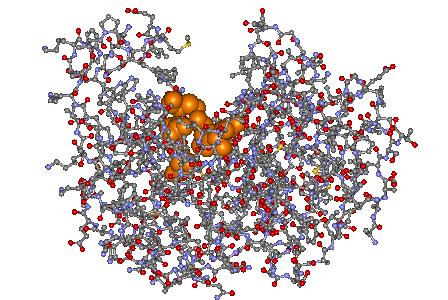
We currently use techniques such as mutagenesis, kinetic analysis and X-ray crystallography to study the role of many important active site residues in class D lactamases. Our over-arching goal is to understand the mechanism by which class D enzymes bind and hydrolyze penicillin, cephalosporin and carbapenem antibiotics. In this structure of the Class D lactamase OXA-1, the orange spheres at the base of the active site groove represent residues critical to the binding and hydrolysis of lactam antibiotics.
In this structure of the Class D lactamase OXA-1, the orange spheres at the base of the active site groove represent residues critical to the binding and hydrolysis of lactam antibiotics.
